
"Cellular Innovation"
What Are Exosomes?
Exosomes are biological vesicles (30-150 nm in diameter) that originate from cells and contain RNA, proteins, and lipids derived from their parent cells. They function as intercellular messengers carrying therapeutic signals between cells because they are capable of crossing biological barriers.
Exosomes derived from stem cells and other cell types contain bioactive molecules including growth factors, mRNA, miRNA, and proteins.
Exosomes can effectively deliver the therapeutic benefits of stem cells without using the cells themselves. They promote tissue regeneration, modulate inflammation, and enhance healing through their rich cargo of signaling molecules and growth factors.

Not All Exosomes Are Created Equal
Exosome are produced in extremely small quantities during the stem cell culture process. Only 0.1% of stem cell media are exosomes.
Therefore, producing exosomes in large quantities while maintaining their functional integrity is technically demanding.
The standard method for obtaining sufficient concentration of exosomes involves repeatedly subculturing large volumes of stem cells, a process known as cell passaging. During this process, the nutrient-rich culture fluid - despite containing valuable regenerative factors is typically discarded.
Repeated cell passaging can lead to genetic and phenotypic changes, reducing the stem cells’ original functionality and degrading the quality of the exosomes they produce.
Maintaining low passage numbers is essential for preserving cell health and producing high-quality exosomes, but achieving consistency at scale remains a major hurdle for most manufacturers.
Our Patented Cell-Culture Process
Precision Filtration + Safety Tests
We use a 0.2 µm filter to effectively remove waste products, bacteria, fungi and particulate contaminants, while preserving the nutrient-rich stem cell conditioned media. This process preserves the naturally secreted cytokines and growth factors known to support regenerative properties. Following filtration, the conditioned media undergoes rigorous testing - including adventitious virus and mycoplasma assays - to ensure it is free of microorganisms.
Characterization and Validation
Our conditioned media is then analyzed using nanoparticle tracking analysis (NTA) and immunoblotting to assess exosome size, morphology, concentration, and specific protein markers. This process ensures our conditioned media contains ~2 billion exosomes per mL- a highly bioactive and concentrated amount of exosomes.
Cryogenic Freeze-Drying
The final step involves advanced lyophilization at -80°C for three days, with sodium hyaluronate and trehalose added as an exosome stabilizer and cryoprotectant to preserve potency and ensure a stable formulation for up to 30 months at room temperature.
Our Result
Superior Quality Exosomes
Fewer cell culture passages mean less replicative stress on the cells, which translates into reduced aging or degradation and, ultimately, higher-quality exosomes.
Increased
Purity
Fewer passages and our optimized growth conditions also minimize opportunities for contamination, ensuring a purer final product.
Enhanced Skin
Penetration by 5x
Lab tests have shown our exosomes are produced with remarkable uniformity in size and stronger cell binding forces, maximizing the delivery of regenerative signals - leading to superior aesthetic applications.
4x Higher
Exosome Yield
Genovia’s patented stem cell culture protocol, provides a faster, more efficient path to therapeutic-scale production without compromising quality.
Industry-Leading Shelf Life
Our robust production methods result in exosomes that maintain their integrity and bioactivity over longer periods. Exosomes produced via this method can retain stability for up to 30 months at room temperature.
Sustainable Quality + Reproducibility
Our exosomes are produced within a state-of-the-art cell and gene therapy manufacturing facility that fully complies with FDA, KFDA and GMP standards. Our operations are governed by rigorous, systematically enforced quality control measures to ensure the highest level of precision, safety, and consistency.
Scientific Validation
Numbers don’t lie. Lab tests show how Genovia Exosomes can help improve the look and feel of the skin and hair. This isn’t hype - it’s science.
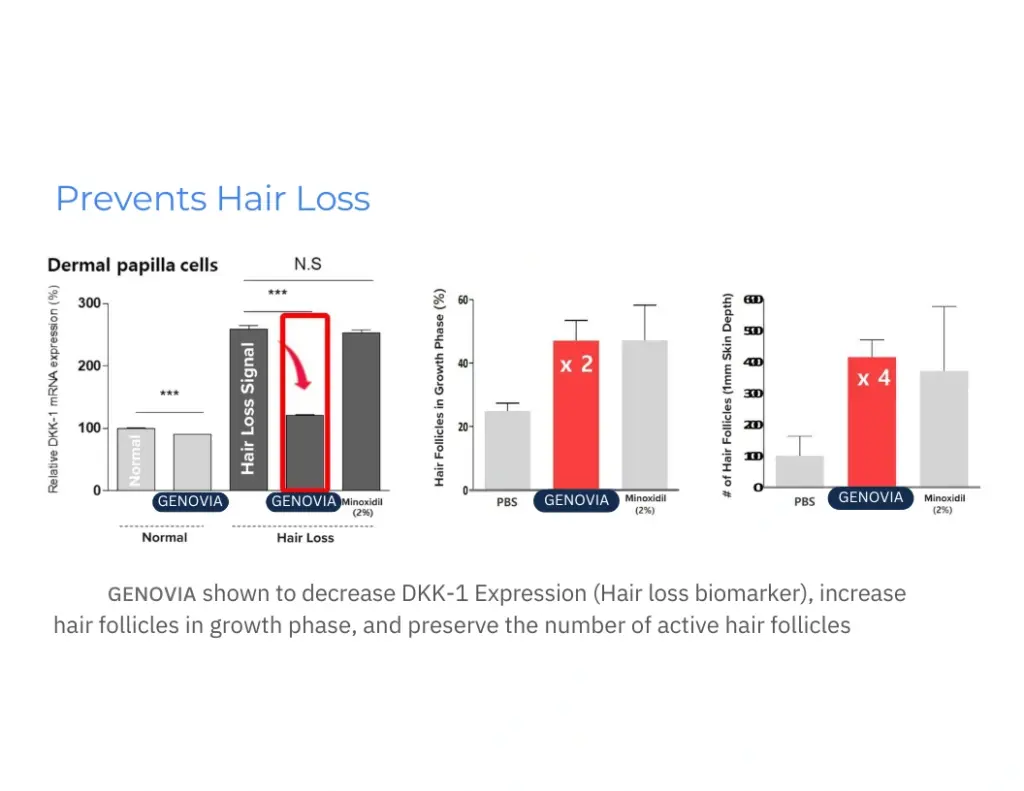
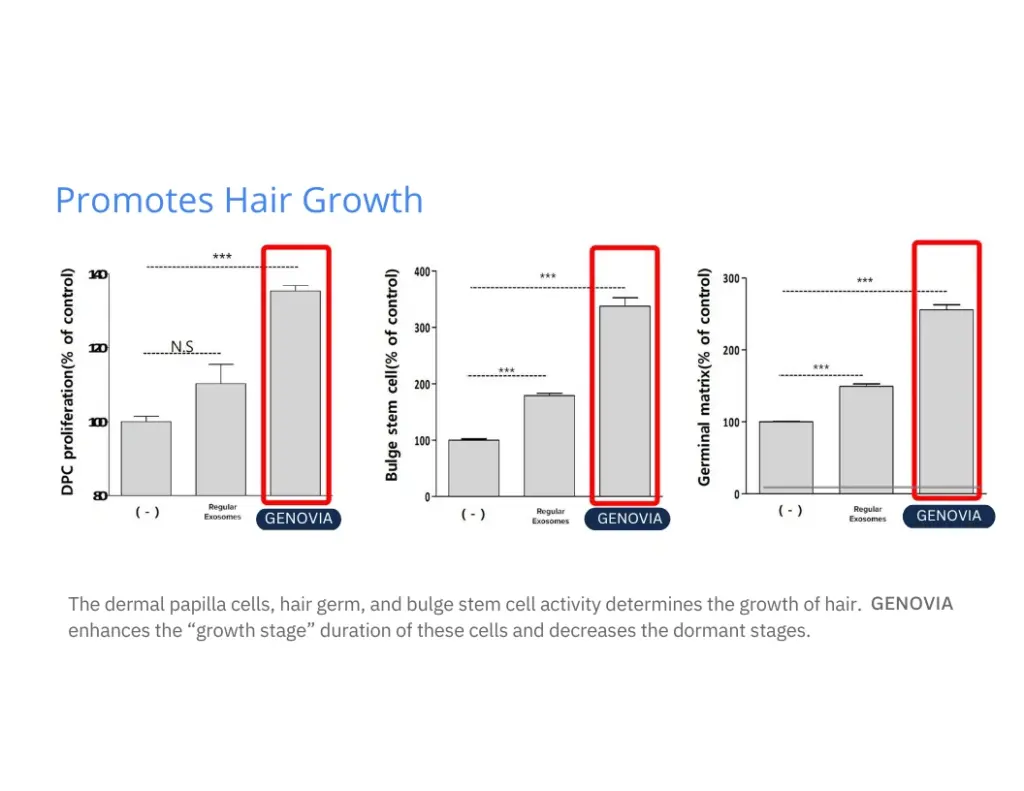
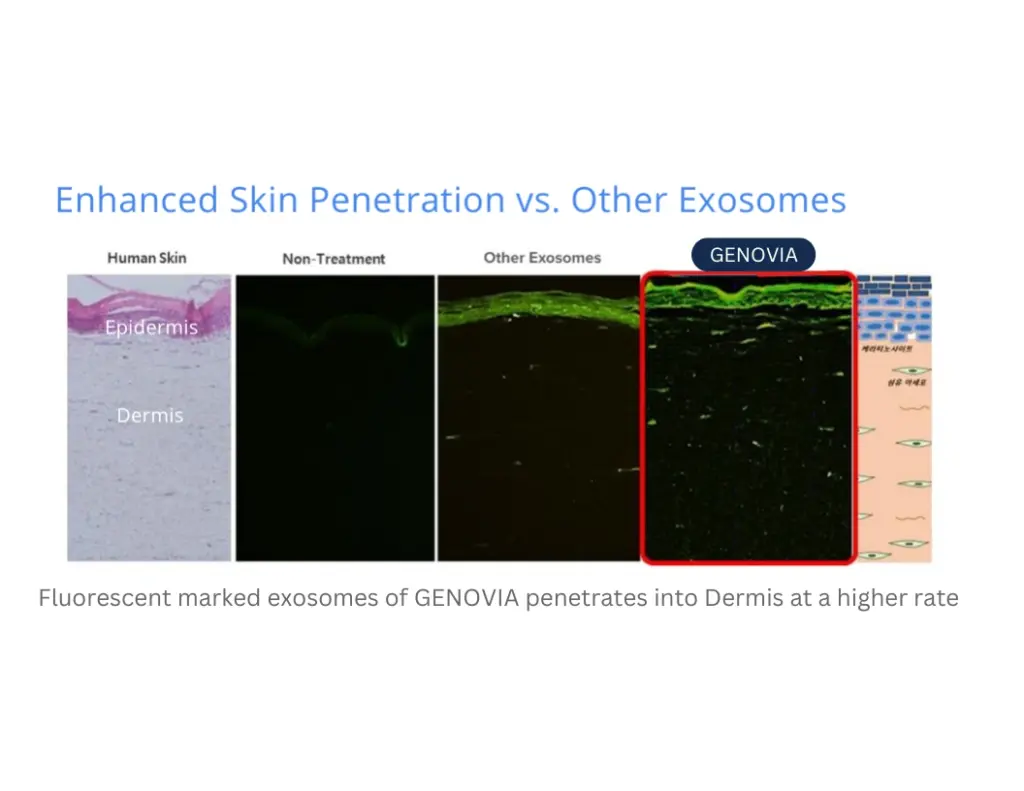

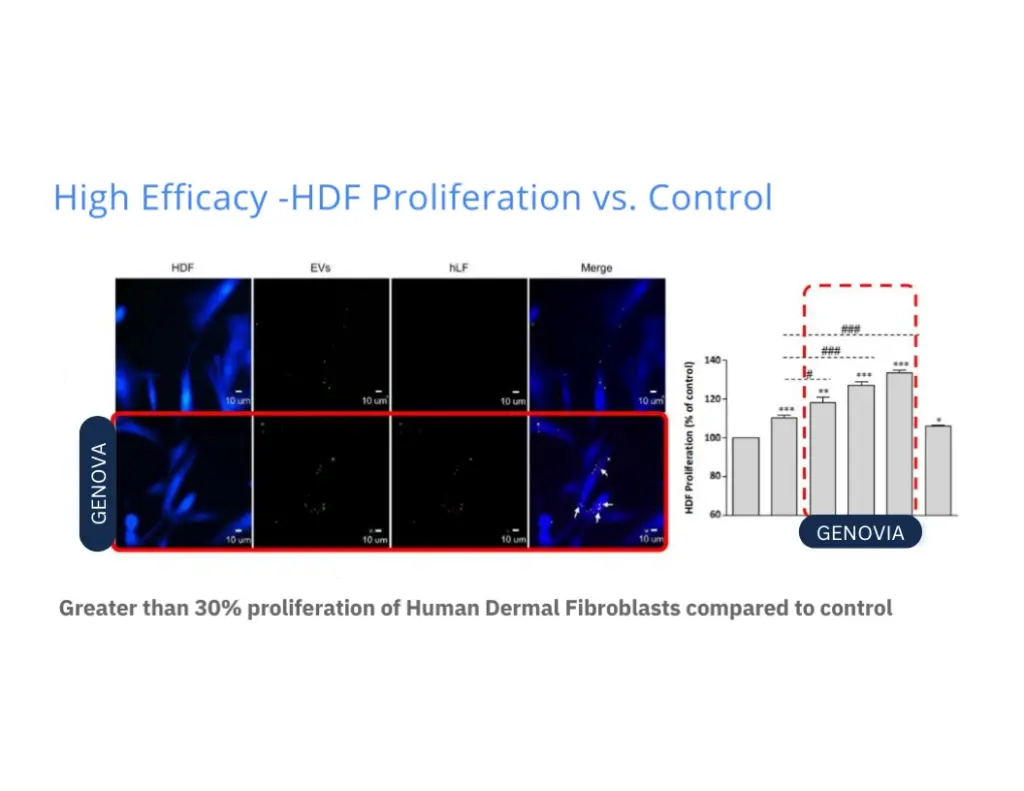
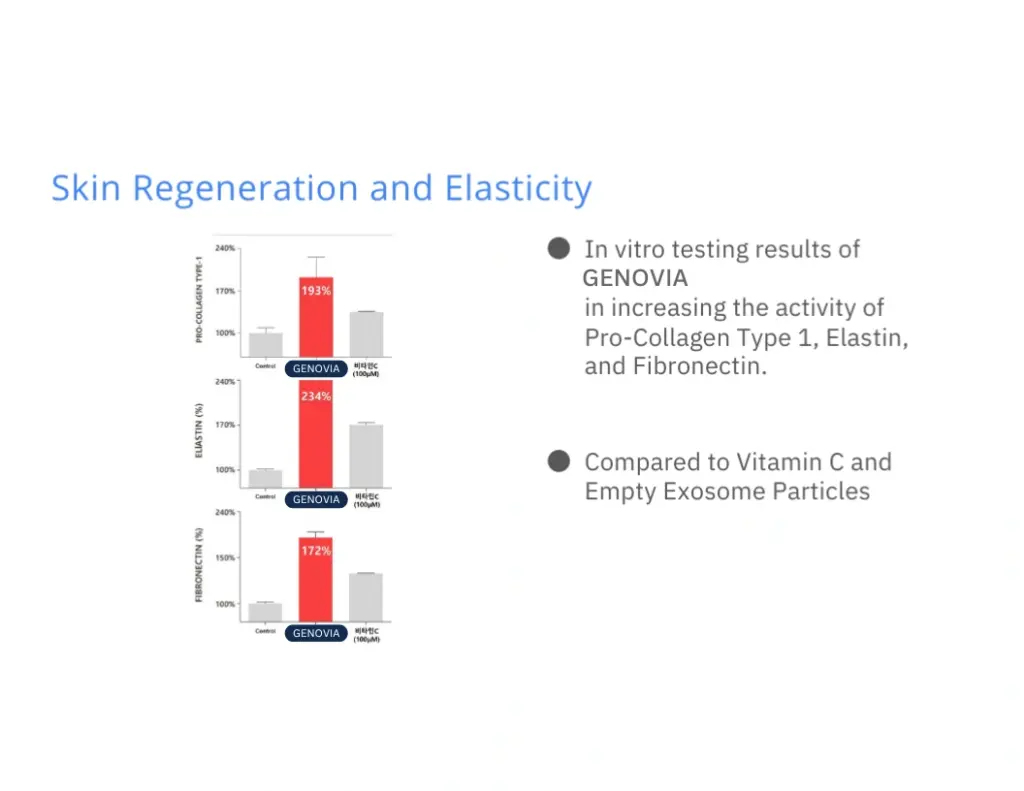
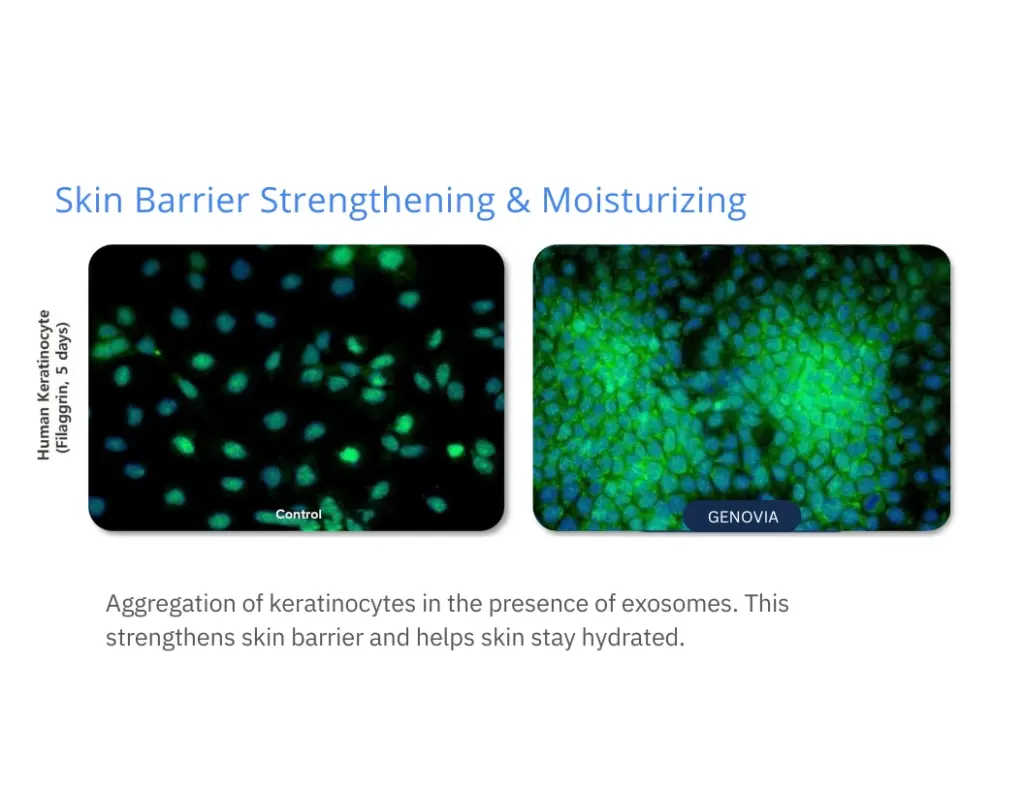
Applications + Use Cases
Fine Lines and Wrinkles
- Improve: the appearance of skin texture.
- Enhance: skin hydration.
- Even: skin tone and brighten complexion.
Anti-Aging
- Improve: appearance of skin firmness.
- Support: appearance of elastic, youthful skin.
- Address: visible signs of aging skin.
Skin Complexion
- Reduce: appearance of redness and irritation.
- Revitalize: skin conditions.
- Enhance: your skin’s natural glow.
Complex Skin Condition
- Reduce: appearance of dark spots and uneven skin tone.
- Minimize: appearance of redness and visible blemishes.
- Smooth: rough or uneven patches on the skin.
Healthier Hair
- Promote: fuller, healthier hair.
- Minimize: hair loss and nourish hair.
Synergistic Effects
- Enhance: effects of other active ingredients (e.g., hyaluronic acid, peptides, phyto PDRN centella).
- Optimize: post treatment results to help skin feel calm, refreshed, and visibly revitalized.
Benefits You’ll Experience
With consistent care using our thoughtfully crafted products, you’ll notice your skin becoming healthier, more balanced, and deeply hydrated.
Enhances skin elasticity while boosting deep hydration.
Diminishes fine lines and reduces visible signs of aging.
Diminishes fine lines and reduces visible signs of aging.
Our Commitment to Safety and Quality
We proudly produce our stem cells in a GMP-certified cell and gene therapy manufacturing facility, following strict FDA and KFDA standards to ensure precision, safety, and consistency.



01
Ethical Stem Cell Recruitment
Single Donor Lots: Each product lot is derived from cells collected from a single donor for consistency and traceability.
Rigorous Cell Therapy Standards: Our donor selection and screening align with recognized cell therapy donor regulations.
Voluntary Donations: All cell donations are made freely and voluntarily.
02
Quality Assurance
Safety Evaluation: Physical assessment of cell structure, pH measurement, and sterility testing.
Purity Assessment: Examination for cellular fragments and confirmation of sterile environment.
Benchmark Evaluation: Protein concentration measurement and quantification of exosomes.
03
Toxicology Testing
Sensitivity Testing: Skin irritation and sensitization.
Oxicology Testing: genotoxicity and phototoxicity.
04
Efficacy Testing
Skin Permeation and Barrier Function Assessment.
Moisture measurement
Evaluation of intended beneficial effects on Anti-aging and skin Brightening
05
Specialized Testing: Healing
First, Create Scratch: A controlled “wound” or scratch is made in a layer of cells.
Then, Incubate Cells: Cells are cultured and allowed time to regenerate and migrate.
Finally, Measure Regeneration: Reduction in the scratch area is measured to assess regenerative potential.
What Experts Are Saying

"I’ve tried various exosomes for hair growth with little to no success-until Biogenomics! After just one session with Biogenomics exosomes, the difference has been astounding: visible hair growth and thickness. Yes, you heard that right—one session! Thank you, Biogenomics, for creating such an effective solution for hair restoration!"
Warda Ali
Founder, WOW Health & Beauty

"Our clients have experienced remarkable improvements in skin health and appearance, thanks to Genovia’s advanced formulations. They report increased confidence and satisfaction with their skin."
April & Jonathan Sigg
Founder, The Laser Lounge Spa

"As an aesthetic practitioner who has utilized countless skin rejuvenation technologies, I’ve found Genovia exosomes to be in a league of their own. My clients see faster, more noticeable improvements in their skin quality. Genovia has quickly become my go-to solution for delivering superior, transformative results."
Lindsay Bright
Founder, Flawless Aesthetics